Blending KTS with UAN
Mixing any liquid potassium source, including KTS®, with an UAN solution will form potassium nitrate, KNO3. Due to KNO3’s low solubility, precipitation will occur causing the need for water. There is a limited amount of KNO3 that can dissolve in a specific amount of water at any given temperature level. In colder environments, KNO3 is less soluble.
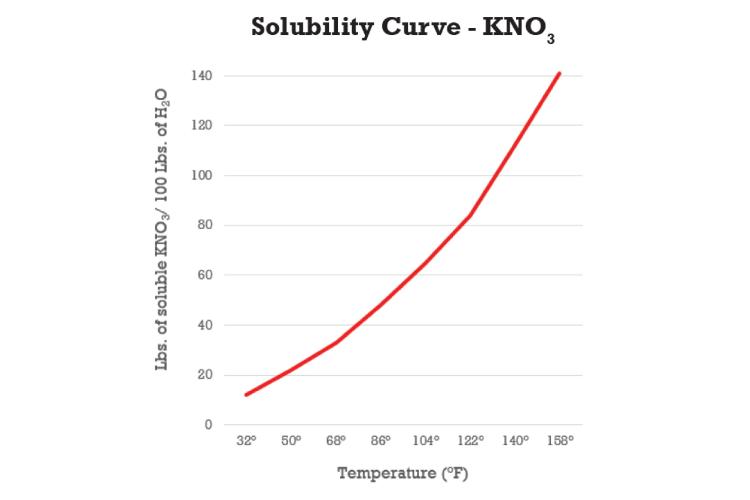
Solubility of KNO3 Explained
Potassium nitrate is sensitive to cold temperatures and can even form crystals at higher temperatures (See graph). The potassium from KTS combines with the nitrate from UAN to form KNO3. As the temperature of the solution decreases, less KNO3 can stay in solution.
For example, at 86ºF (30ºC), approximately 48 pounds of KNO3 can be dissolved in 100 pounds of water. At 50ºF (10ºC), only 21 pounds can be dissolved in 100 pounds of water, less than half. This drop in solubility is the reason why crystals form in these types of blends.
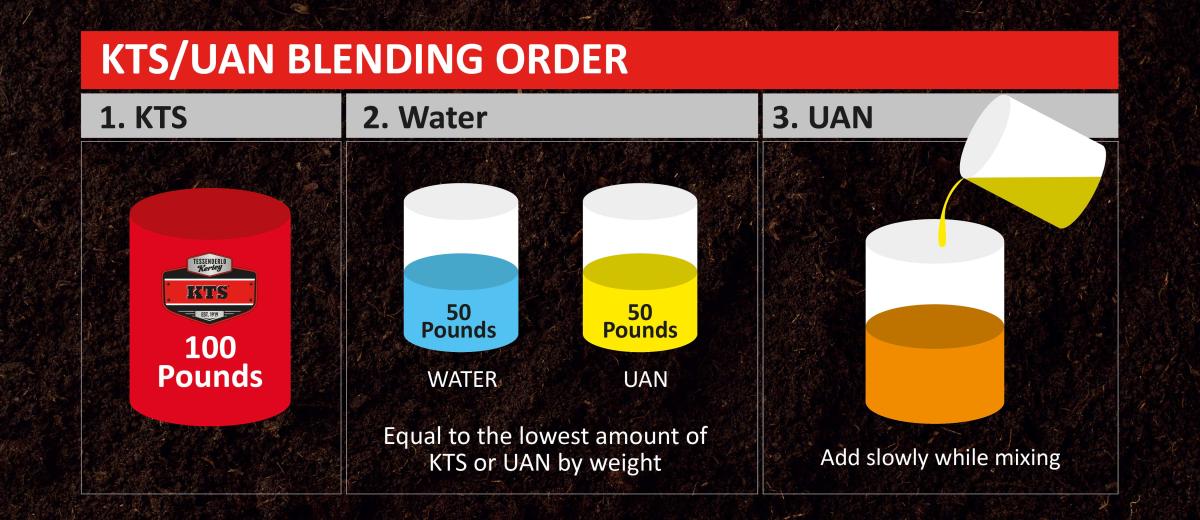
Learn more about KTSBlending Order: KTS and UAN
When mixing KTS with UAN solution, add water equal in weight to KTS or UAN, whichever has the smaller quantity.
For example, if mixing 100 pounds of KTS with 50 pounds of UAN, add at least 50 pounds of water. See figure below.
It’s important to note, in cold weather, adding additional water or increasing the temperature of the blend may be needed.